Magnetic Resonance Imaging
From Wikipedia, the free encyclopedia.
Magnetic resonance imaging (MRI), nuclear magnetic resonance imaging (NMRI), or magnetic resonance tomography (MRT) is a medical imaging technique used in radiology to visualize internal structures of the body in detail. MRI makes use of the property of nuclear magnetic resonance (NMR) to image nuclei of atoms inside the body. MRI can create more detailed images of the human body than are possible with X-rays.
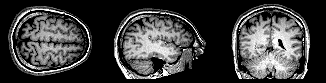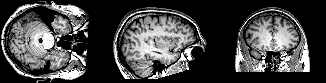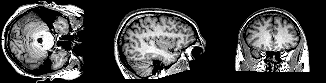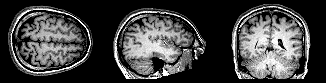 |
| Sequential sections from an MRI of the brain, concurrently showing slices through transverse, sagittal, and coronal planes (left to right). |
MRI provides good contrast between the different soft tissues of the body, which makes it especially useful in imaging the brain, muscles, the heart, and cancers compared with other medical imaging techniques such as computed tomography (CT) or X-rays. Unlike CT scans or traditional X-rays, MRI does not use ionizing radiation.
How MRI Works
| Medical MRI Scanner |
Information about the origin of the signal in 3D space can be learned by applying additional magnetic fields during the scan. These additional magnetic fields can be used to generate detectable signals only from specific locations in the body (spatial excitation) and/or to make magnetization at different spatial locations precess at different frequencies, which enables k-space encoding of spatial information. The 3D images obtained in MRI can be rotated along arbitrary orientations and manipulated by the doctor to be better able to detect tiny changes of structures within the body. These fields, generated by passing electric currents through gradient coils, make the magnetic field strength vary depending on the position within the magnet. Because this makes the frequency of the released radio signal also dependent on its origin in a predictable manner, the distribution of protons in the body can be mathematically recovered from the signal, typically by the use of inverse Fourier transform.
Protons in different tissues return to their equilibrium state at different relaxation rates. Different tissue variables, including spin density, T1 and T2 relaxation times, and flow and spectral shifts, can be used to construct images. By changing the settings on the scanner, this effect is used to create contrast between different types of body tissue or between other properties, as in fMRI and diffusion MRI.
MRI is used to image every part of the body, and is particularly useful for tissues with many hydrogen nuclei and little density contrast, such as the brain, muscle, connective tissue and most tumors.
Magnetic Field
MRI scans require a magnetic field with two properties, uniform field density and strength. The magnetic field cannot vary more than 1/10,000 of 1% and field strength ranges (depending on the scanner) from 0.2 to 3 teslas in strength in scanners currently used clinically, with research scanners investigating higher field strengths such as 7 teslas. The lower field strengths can be achieved with permanent magnets, which are often used in "open" MRI scanners for claustrophobic patients. Higher field strengths can be achieved only with superconducting magnets. An MRI with a 3.0 tesla strength magnet may be referred to as a "3-T MRI" or "3-tesla MRI"
Since the gradient coils are within the bore of the scanner, there are large forces between them and the main field coils, producing most of the hammering noise that is heard during operation. Without efforts to damp this noise, it can approach 130 decibels (dB) with strong fields (see also the subsection on acoustic noise). Contrast agents and implants
MRI contrast agents may be injected intravenously to enhance the appearance of blood vessels, tumors or inflammation. Contrast agents may also be directly injected into a joint in the case of arthrograms: MRI images of joints. Unlike CT, MRI uses no ionizing radiation and is generally a very safe procedure. Nonetheless the strong magnetic fields and radio pulses can affect metal implants, including cochlear implants and cardiac pacemakers. There are many electronically activated devices that have approval from the US FDA to permit MRI procedures in patients under highly specific MRI conditions (see www.MRIsafety.com). In the case of cochlear implants, the US FDA has approved some implants for MRI compatibility. In the case of cardiac pacemakers, the results can sometimes be lethal, so patients with such implants are generally not eligible for MRI.
Prepolarized MRI
In 2001, a research team at Stanford invented a new technique which came to be called "Prepolarized MRI" or PMRI. The team demonstrated that the magnets do not have to be both uniform and strong. Rather, two magnets can be used together, where one is strong and the other one is uniform.
The first magnet in a PMRI scanner is strong, but not uniform. This magnet creates a very strong magnetic field which varies in uniformity by as much as 40%. This is the "prepolarize" component. A second much weaker (requiring only the electric power necessary to run two hairdryers) but far more precise magnet then creates a homogeneous magnetic field. These two magnets can be ordinary copper wound magnets, greatly lowering the cost of an MRI scanner. Because the magnetic field is "tuned" by the second magnet, a PMRI scan can be obtained immediately adjacent to a metal prosthetic, unlike an MRI scan.
History
In 1952, Herman Carr produced a one-dimensional MRI image as reported in his Harvard PhD thesis. In the Soviet Union, Vladislav Ivanov filed (in 1960) a document with the USSR State Committee for Inventions and Discovery at Leningrad for a Magnetic Resonance Imaging device, although this was not approved until the 1970s.
 |
| Raymond Damadian's "Apparatus and method for detecting cancer in tissue." |
The National Science Foundation notes, "The patent included the idea of using NMR to 'scan' the human body to locate cancerous tissue." However, it did not describe a method for generating pictures from such a scan or precisely how such a scan might be done. Meanwhile, Paul Lauterbur expanded on Carr's technique and developed a way to generate the first MRI images, in 2D and 3D, using gradients. In 1973, Lauterbur published the first nuclear magnetic resonance image and the first cross-sectional image of a living mouse in January 1974. In the late 1970s, Peter Mansfield, a physicist and professor at the University of Nottingham, England, developed a mathematical technique that would allow scans to take seconds rather than hours and produce clearer images than Lauterbur had. Damadian, along with Larry Minkoff and Michael Goldsmith, performed the first MRI body scan of a human being on July 3, 1977, studies which they published in 1977. and in 1979 Richard S. Likes filed patent *4,307,343.
In 1980 Paul Bottomley joined the GE Research Center in Schenectady, NY, and his team ordered the highest field-strength magnet then available — a 1.5T system — and built the first high-field and overcame problems of coil design, RF penetration and signal-to-noise ratio to build the first whole-body MRI/MRS scanner. The results translated into the highly successful 1.5T MRI product-line, with over 20,000 systems in use today. Bottomley performed the first localized MRS in the human heart and brain. After starting a collaboration on heart applications with Robert Weiss at Johns Hopkins, Bottomley returned to the university in 1994 as Russell Morgan Professor and director of the MR Research Division. Although MRI is most commonly performed at 1.5 T, higher fields such as 3T are gaining more popularity because of their increased sensitivity and resolution. In research laboratories, human studies have been performed at up to 9.4 T and animal studies have been performed at up to 21.1T.
2003 Nobel Prize
Reflecting the fundamental importance and applicability of MRI in medicine, Paul Lauterbur of the University of Illinois at Urbana-Champaign and Sir Peter Mansfield of the University of Nottingham were awarded the 2003 Nobel Prize in Physiology or Medicine for their "discoveries concerning magnetic resonance imaging". The Nobel citation acknowledged Lauterbur's insight of using magnetic field gradients to determine spatial localization, a discovery that allowed rapid acquisition of 2D images. Mansfield was credited with introducing the mathematical formalism and developing techniques for efficient gradient utilization and fast imaging. The actual research that won the prize was done almost 30 years before, while Paul Lauterbur was at Stony Brook University in New York.
The award was vigorously protested by Raymond Vahan Damadian, founder of FONAR Corporation, who claimed that he invented the MRI, and that Lauterbur and Mansfield had merely refined the technology. A group called "The Friends of Raymond Damadian" (formed by Damadian's company, FONAR), took out full-page advertisements in the New York Times and The Washington Post entitled "The Shameful Wrong That Must Be Righted", demanding that he be awarded at least a share of the Nobel Prize.
Applications
In clinical practice, MRI is used to distinguish pathologic tissue (such as a brain tumor) from normal tissue. One advantage of an MRI scan is that it is harmless to the patient. It uses strong magnetic fields and non-ionizing electromagnetic fields in the radio frequency range, unlike CT scans and traditional X-rays, which both use ionizing radiation.
While CT provides good spatial resolution (the ability to distinguish two separate structures at a small distance from each other), MRI provides comparable resolution with far better contrast resolution (the ability to distinguish the differences between two similar but not identical tissues). The basis of this ability is the complex library of pulse sequences that the modern medical MRI scanner includes, each of which is optimized to provide image contrast based on the chemical sensitivity of MRI.
 |
| Effects of TR and TE on MR signal. |
The typical MRI examination consists of 5–20 sequences, each of which is chosen to provide a particular type of information about the subject tissues. This information is then synthesized by the interpreting physician.
Basic MRI Scans
T1-weighted MRI
T1-weighted scans refer to a set of standard scans that depict differences in the spin-lattice (or T1) relaxation time of various tissues within the body. T1 weighted images can be acquired using either spin echo or gradient-echo sequences. T1-weighted contrast can be increased with the application of an inversion recovery RF pulse. Gradient-echo based T1-weighted sequences can be acquired very rapidly because of their ability to use short inter-pulse repetition times (TR). T1-weighted sequences are often collected before and after infusion of T1-shortening MRI contrast agents. In the brain T1-weighted scans provide appreciable contrast between gray and white matter. In the body, T1 weighted scans work well for differentiating fat from water—with water appearing darker and fat brighter.
T2-weighted MRI
T2-weighted scans refer to a set of standard scans that depict differences in the spin-spin (or T2) relaxation time of various tissues within the body. Like the T1-weighted scan, fat is differentiated from water, but in this case fat shows darker, and water lighter. For example, in the case of cerebral and spinal study, the CSF (cerebrospinal fluid) will be lighter in T2-weighted images. These scans are therefore particularly well suited to imaging edema, with long TE and long TR. Because the spin echo sequence is less susceptible to inhomogeneities in the magnetic field, these images have long been a clinical workhorse.
T2*-weighted MRI
T2* (pronounced "T 2 star") weighted scans use a gradient echo (GRE) sequence, with long TE and long TR. The gradient echo sequence used does not have the extra refocusing pulse used in spin echo so it is subject to additional losses above the normal T2 decay (referred to as T2′), these taken together are called T2*. This also makes it more prone to susceptibility losses at air/tissue boundaries, but can increase contrast for certain types of tissue, such as venous blood.
Spin Density Weighted MRI
Spin density, also called proton density, weighted scans try to have no contrast from either T2 or T1 decay, the only signal change coming from differences in the amount of available spins (hydrogen nuclei in water). It uses a spin echo or sometimes a gradient echo sequence, with short TE and long TR.
Specialized MRI Scans
Diffusion MRI
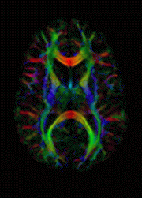 |
| DTI image |
The recent development of diffusion tensor imaging (DTI) enables diffusion to be measured in multiple directions and the fractional anisotropy in each direction to be calculated for each voxel. This enables researchers to make brain maps of fiber directions to examine the connectivity of different regions in the brain (using tractography) or to examine areas of neural degeneration and demyelination in diseases like multiple sclerosis.
Another application of diffusion MRI is diffusion-weighted imaging (DWI). Following an ischemic stroke, DWI is highly sensitive to the changes occurring in the lesion. It is speculated that increases in restriction (barriers) to water diffusion, as a result of cytotoxic edema (cellular swelling), is responsible for the increase in signal on a DWI scan. The DWI enhancement appears within 5–10 minutes of the onset of stroke symptoms (as compared with computed tomography, which often does not detect changes of acute infarct for up to 4–6 hours) and remains for up to two weeks. Coupled with imaging of cerebral perfusion, researchers can highlight regions of "perfusion/diffusion mismatch" that may indicate regions capable of salvage by reperfusion therapy.
Like many other specialized applications, this technique is usually coupled with a fast image acquisition sequence, such as echo planar imaging sequence.
Magnetization Transfer MRI
Magnetization transfer (MT) refers to the transfer of longitudinal magnetization from free water protons to hydration water protons in NMR and MRI.
In magnetic resonance imaging of molecular solutions, such as protein solutions, two types of water molecules, free (bulk) and hydration (bound), are found. Free water protons have faster average rotational frequency and hence fewer fixed water molecules that may cause local field inhomogeneity. Because of this uniformity, most free water protons have resonance frequency lying narrowly around the normal proton resonance frequency of 63 MHz (at 1.5 teslas). This also results in slower transverse magnetization dephasing and hence longer T2. Conversely, hydration water molecules are slowed down by interaction with solute molecules and hence create field inhomogeneities that lead to a wider resonance frequency spectrum.
In free liquids, protons, which may be viewed classically as small magnetic dipoles, exhibit translational and rotational motions. These moving dipoles disturb the surrounding magnetic field. However, on long enough time-scales (which may be nanoseconds) the average field caused by the motion of protons is zero. This is known as "motional averaging" or narrowing and is characteristic of protons moving freely in liquid. On the other hand, protons bound to macromolecules, such as proteins, tend to have a fixed orientation and so the average magnetic field in close proximity to such structures does not average to zero. The result is a spatial pattern in the magnetic field that gives rise to a residual dipolar coupling (range of precession frequencies) for the protons experiencing the magnetic field. The wide frequency distribution appears as a broad spectrum that may be several kHz wide. The net signal from these protons disappears very quickly, in inverse proportion to the width, due to the loss of coherence of the spins, i.e., T2 relaxation. Due to exchange mechanisms such as spin transfer or proton chemical exchange, the (incoherent) spins bound to the macromolecules continually switch places with (coherent) spins in the bulk media and establish a dynamic equilibrium.
Magnetization transfer: Although there is no measurable signal from the bound spins, or the bound spins that exchange into the bulk media, their longitudinal magnetization is preserved and may recover only via the relatively slow process of T1 relaxation. If the longitudinal magnetization of just the bound spins can be altered, then the effect can be measured in the spins of the bulk media due to the exchange processes. The magnetization transfer sequence applies RF saturation at a frequency that is far off resonance for the narrow line of bulk water but still on resonance for the bound protons with a spectral linewidth of kHz. This causes saturation of the bound spins which exchange into the bulk water, resulting in a loss of longitudinal magnetization and hence signal decrease in the bulk water. This provides an indirect measure of macromolecular content in tissue. Implementation of magnetization transfer involves choosing suitable frequency offsets and pulse shapes to saturate the bound spins sufficiently strongly, within the safety limits of specific absorption rate for RF irradiation.
T1rho MRI
T1ρ (T1rho): Molecules have a kinetic energy that is a function of the temperature and is expressed as translational and rotational motions, and by collisions between molecules. The moving dipoles disturb the magnetic field but are often extremely rapid so that the average effect over a long time-scale may be zero. However, depending on the time-scale, the interactions between the dipoles do not always average away. At the slowest extreme the interaction time is effectively infinite and occurs where there are large, stationary field disturbances (e.g., a metallic implant). In this case the loss of coherence is described as a "static dephasing". T2* is a measure of the loss of coherence in an ensemble of spins that includes all interactions (including static dephasing). T2 is a measure of the loss of coherence that excludes static dephasing, using an RF pulse to reverse the slowest types of dipolar interaction. There is in fact a continuum of interaction time-scales in a given biological sample, and the properties of the refocusing RF pulse can be tuned to refocus more than just static dephasing. In general, the rate of decay of an ensemble of spins is a function of the interaction times and also the power of the RF pulse. This type of decay, occurring under the influence of RF, is known as T1ρ. It is similar to T2 decay but with some slower dipolar interactions refocused, as well as static interactions, hence T1ρ≥T2.
Fluid Attenuated Inversion Recovery (FLAIR)
Fluid Attenuated Inversion Recovery (FLAIR) is an inversion-recovery pulse sequence used to nullify the signal from fluids. For example, it can be used in brain imaging to suppress cerebrospinal fluid (CSF) so as to bring out periventricular hyperintense lesions, such as multiple sclerosis (MS) plaques. By carefully choosing the inversion time TI (the time between the inversion and excitation pulses), the signal from any particular tissue can be suppressed.
Magnetic Resonance Angiography
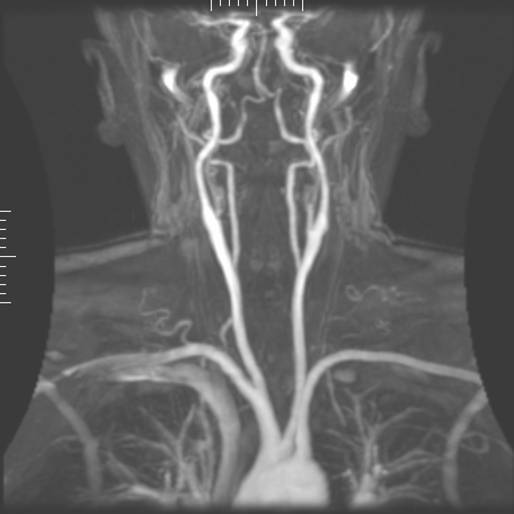 |
| Magnetic resonance angiography |
Magnetic Resonance Gated Intracranial CSF Dynamics (MR-GILD)
Magnetic resonance gated intracranial cerebrospinal fluid (CSF) or liquor dynamics (MR-GILD) technique is an MR sequence based on bipolar gradient pulse used to demonstrate CSF pulsatile flow in ventricles, cisterns, the aqueduct of Sylvius, and the entire intracranial CSF pathway. It is a method for analyzing CSF circulatory system dynamics in patients with CSF obstructive lesions such as normal pressure hydrocephalus. It also allows visualization of both arterial and venous pulsatile blood flow in vessels without the use of contrast agents.
Magnetic Resonance Spectroscopy
Magnetic resonance spectroscopy (MRS) is used to measure the levels of different metabolites in body tissues. The MR signal produces a spectrum of resonances that corresponds to different molecular arrangements of the isotope being "excited". This signature is used to diagnose certain metabolic disorders, especially those affecting the brain, and to provide information on tumor metabolism.
Magnetic resonance spectroscopic imaging (MRSI) combines both spectroscopic and imaging methods to produce spatially localized spectra from within the sample or patient. The spatial resolution is much lower (limited by the available SNR), but the spectra in each voxel contains information about many metabolites. Because the available signal is used to encode spatial and spectral information, MRSI requires high SNR achievable only at higher field strengths (3 T and above).
Functional MRI
 |
| A fMRI scan showing regions of activation in orange, including the primary visual cortex (V1, BA17). |
While BOLD signal analysis is the most common method employed for neuroscience studies in human subjects, the flexible nature of MR imaging provides means to sensitize the signal to other aspects of the blood supply. Alternative techniques employ arterial spin labeling (ASL) or weighting the MRI signal by cerebral blood flow (CBF) and cerebral blood volume (CBV). The CBV method requires injection of a class of MRI contrast agents that are now in human clinical trials. Because this method has been shown to be far more sensitive than the BOLD technique in preclinical studies, it may potentially expand the role of fMRI in clinical applications. The CBF method provides more quantitative information than the BOLD signal, albeit at a significant loss of detection sensitivity.
Real-time MRI
Real-time MRI refers to the continuous monitoring ("filming") of moving objects in real time. While many different strategies have been developed over the past two decades, a recent development reported a real-time MRI technique based on radial FLASH and iterative reconstruction that yields a temporal resolution of 20 to 30 milliseconds for images with an in-plane resolution of 1.5 to 2.0 mm. The new method promises to add important information about diseases of the joints and the heart. In many cases MRI examinations may become easier and more comfortable for patients.
Interventional MRI
The lack of harmful effects on the patient and the operator make MRI well-suited for "interventional radiology", where the images produced by a MRI scanner are used to guide minimally invasive procedures. Of course, such procedures must be done without any ferromagnetic instruments.
A specialized growing subset of interventional MRI is that of intraoperative MRI in which the MRI is used in the surgical process. Some specialized MRI systems have been developed that allow imaging concurrent with the surgical procedure. More typical, however, is that the surgical procedure is temporarily interrupted so that MR images can be acquired to verify the success of the procedure or guide subsequent surgical work.
Radiation Therapy Simulation
Because of MRI's superior imaging of soft tissues, it is now being utilized to specifically locate tumors within the body in preparation for radiation therapy treatments. For therapy simulation, a patient is placed in a specific, reproducible, body position and scanned. The MRI system then computes the precise location, shape and orientation of the tumor mass, correcting for any spatial distortion inherent in the system. The patient is then marked or tattooed with points that, when combined with the specific body position, permits precise triangulation for radiation therapy.
Current Density Imaging
Current density imaging (CDI) endeavors to use the phase information from images to reconstruct current densities within a subject. Current density imaging works because electrical currents generate magnetic fields, which in turn affect the phase of the magnetic dipoles during an imaging sequence.
Magnetic Resonance Guided Focused Ultrasound
In MRgFUS therapy, ultrasound beams are focused on a tissue—guided and controlled using MR thermal imaging—and due to the significant energy deposition at the focus, temperature within the tissue rises to more than 65 °C (150 °F), completely destroying it. This technology can achieve precise ablation of diseased tissue. MR imaging provides a three-dimensional view of the target tissue, allowing for precise focusing of ultrasound energy. The MR imaging provides quantitative, real-time, thermal images of the treated area. This allows the physician to ensure that the temperature generated during each cycle of ultrasound energy is sufficient to cause thermal ablation within the desired tissue and if not, to adapt the parameters to ensure effective treatment.
Multinuclear Imaging
Hydrogen is the most frequently imaged nucleus in MRI because it is present in biological tissues in great abundance, and because its high gyromagnetic ratio gives a strong signal. However, any nucleus with a net nuclear spin could potentially be imaged with MRI. Such nuclei include helium-3, lithium-7, carbon-13, fluorine-19, oxygen-17, sodium-23, phosphorus-31 and xenon-129. 23Na and 31P are naturally abundant in the body, so can be imaged directly. Gaseous isotopes such as 3He or 129Xe must be hyperpolarized and then inhaled as their nuclear density is too low to yield a useful signal under normal conditions. 17O and 19F can be administered in sufficient quantities in liquid form (e.g. 17O-water) that hyperpolarization is not a necessity.
Multinuclear imaging is primarily a research technique at present. However, potential applications include functional imaging and imaging of organs poorly seen on 1H MRI (e.g., lungs and bones) or as alternative contrast agents. Inhaled hyperpolarized 3He can be used to image the distribution of air spaces within the lungs. Injectable solutions containing 13C or stabilized bubbles of hyperpolarized 129Xe have been studied as contrast agents for angiography and perfusion imaging. 31P can potentially provide information on bone density and structure, as well as functional imaging of the brain. Multinuclear imaging holds the potential to chart the distribution of lithium in the human brain, this element finding use as an important drug for those with conditions such as bipolar disorder.
Susceptibility Weighted Imaging (SWI)
Susceptibility weighted imaging (SWI), is a new type of contrast in MRI different from spin density, T1, or T2 imaging. This method exploits the susceptibility differences between tissues and uses a fully velocity compensated, three dimensional, RF spoiled, high-resolution, 3D gradient echo scan. This special data acquisition and image processing produces an enhanced contrast magnitude image very sensitive to venous blood, hemorrhage and iron storage. It is used to enhance the detection and diagnosis of tumors, vascular and neurovascular diseases (stroke and hemorrhage, multiple sclerosis, Alzheimer's), and also detects traumatic brain injuries that may not be diagnosed using other methods.
Other Specialized MRI Techniques
New methods and variants of existing methods are often published when they are able to produce better results in specific fields. Examples of these recent improvements are T2*-weighted turbo spin-echo (T2 TSE MRI), double inversion recovery MRI (DIR-MRI) or phase-sensitive inversion recovery MRI (PSIR-MRI), all of them able to improve imaging of brain lesions. Another example is MP-RAGE (magnetization-prepared rapid acquisition with gradient echo), which improves images of multiple sclerosis cortical lesions.
Molecular Imaging of Disease Biomarkers by MRI
MRI has the advantages of having very high spatial resolution and is very adept at morphological imaging and functional imaging. MRI does have several disadvantages though. First, MRI has a sensitivity of around 10−3 mol/L to 10−5 mol/L which, compared to other types of imaging, can be very limiting. This problem stems from the fact that the difference between atoms in the high energy state and the low energy state is very small. For example, at 1.5 teslas, a typical field strength for clinical MRI, the difference between high and low energy states is approximately 9 molecules per 2 million. Improvements to increase MR sensitivity include increasing magnetic field strength, and hyperpolarization via optical pumping or dynamic nuclear polarization. There are also a variety of signal amplification schemes based on chemical exchange that increase sensitivity.
To achieve molecular imaging of disease biomarkers using MRI, targeted MRI contrast agents with high specificity and high relaxivity (sensitivity) are required. To date, many studies have been devoted to developing targeted-MRI contrast agents to achieve molecular imaging by MRI. Commonly, peptides, antibodies, or small ligands, and small protein domains, such as HER-2 affibodies, have been applied to achieve targeting. To enhance the sensitivity of the contrast agents, these targeting moieties are usually linked to high payload MRI contrast agents or MRI contrast agents with high relaxivities.
Portable Instruments
Portable magnetic resonance instruments are available for use in education and field research. Using the principles of Earth's field NMR, they have no powerful polarizing magnet, so that such instruments can be small and inexpensive. Some can be used for both EFNMR spectroscopy and MRI imaging. The low strength of the Earth's field results in poor signal to noise ratios (SNR), requiring long scan times to capture spectroscopic data or build up MRI images. However, the extremely low noise floor of SQUID-based MRI detectors and the low density of thermal noise in the low-frequency operating range (tens of kiloHertz) may result in usable SNR approaching that of mid-field conventional instruments. Further, the ultra-low field technologies enable electron spin resonance detection, and potentially imaging, at safe operating frequencies.
Research with atomic magnetometers has addressed the possibility of cheap and portable MRI instruments without a large magnet.
MRI Versus CT
The use of X-rays, a type of ionizing radiation, in computed tomography (CT) allows for examination of tissues composed of elements of a higher atomic number than the surrounding tissues. MRI, in contrast, uses non-ionizing radio frequency (RF) signals to acquire images and is best suited for soft tissue (although MRI can also be used to visualize bones, teeth and even fossils).
Since CT scans use ionizing radiation (X-rays) to produce images, there is a risk of damage to DNA that can subsequently cause cancer. In 2007, it was estimated that 0.4% of current cancers in the United States were due to CTs performed in the past, and that in the future this figure may increase to as high as 1.5–2% based on past rates of CT usage. Unlike CT, MRI does not use ionizing radiation, although it is associated with other risks.
Contrast in CT images is generated purely by X-ray attenuation, while a variety of properties may be used to generate contrast in MR images. By varying the scanning parameters, tissue contrast can be altered to enhance different features in an image (see Applications for more details). Both CT and MR images may be enhanced by using contrast agents. Contrast agents for CT contain elements of a high atomic number relative to the tissue being investigated, such as iodine or barium, while contrast agents (such as gadolinium and manganese) for MRI have paramagnetic properties that are used to alter tissue relaxation times. Commonly used MRI contrast agents may be contraindicated in people with significant permanent or transient kidney dysfunction.
CT and MRI scanners are able to generate multiple two-dimensional cross-sections (tomographs, or "slices") of tissue and three-dimensional reconstructions. MRI can generate cross-sectional images in any plane (including oblique planes). In the past, CT was limited to acquiring images in the axial plane (or near axial plane), and so these images were called Computed Axial Tomography scans (CAT scans). However, the development of multi-detector CT scanners with near-isotropic resolution allows the CT scanner to produce data that can be retrospectively reconstructed in any plane with minimal loss of image quality. For purposes of tumor detection and identification in the brain, MRI is generally superior. However, in the case of solid tumors of the abdomen and chest, CT is often preferred as it less affected by motion artifacts. Furthermore, CT usually is more widely available, faster, and less expensive.
MRI is also best suited for cases when a patient is to undergo several exams in the short term, since it does not expose the patient to the hazards of ionizing radiation. However MRI is usually contraindicated if the patient has any type of medical implant, such as vagus nerve stimulators, implantable cardioverter-defibrillators, loop recorders, insulin pumps, cochlear implants, deep brain stimulators, metallic foreign bodies (e.g., shrapnel or shell fragments), or metallic implants such as surgical prostheses. These devices can malfunction or heat up during an MRI scan, so CT scans are considered the safer option for these patients.
Economics of MRI
Standard 1.5 tesla MRI scanners used to cost between US$1 million and US$1.5 million. Standard 3.0 tesla MRI scanners would often cost between US$2 million and US$2.3 million. Construction of MRI suites could cost up to US$500,000 or more, depending on project scope. PMRI scanners today now cost a great deal less, around US$50,000.
 |
| Looking through an MRI scanner. |
In the US, the Deficit Reduction Act of 2005 significantly reduced reimbursement rates paid by federal insurance programs for the equipment component of many scans, shifting the economic landscape. Many private insurers have followed suit.
In the United States, an MRI of the brain with and without contrast billed to Medicare Part B entails, on average, a technical payment of US$403 and a separate payment to the radiologist of US$93. In France, the cost of an MRI exam is approximately 150 euros. This covers three basic scans including one with an intravenous contrast agent as well as a consultation with the technician and a written report to the patient's physician. In Japan, the cost of an MRI examination (excluding the cost of contrast material and films) ranges from US$155 to US$180, with an additional radiologist professional fee of US$17. In India, the cost of an MRI examination including the fee for the radiologist's opinion comes to around Rs 3000–4000 (US$50–60), excluding the cost of contrast material.
Safety
A number of features of MRI scanning can give rise to risks. These include:
- Powerful magnetic fields
- Radio waves
- Cryogenic liquids
- Noise
- Claustrophobia.
Overuse
Medical societies issue guidelines for when physicians should use MRI on patients and recommend against overuse. MRI can detect health problems or confirm a diagnosis, but medical societies often recommend that MRI not be the first procedure for creating a plan to diagnose or manage a patient's complaint. A common case is to use MRI to seek a cause of low back pain; the American College of Physicians, for example, recommends against this procedure as unlikely to result in a positive outcome for the patient. Nevertheless, MRI has the advantage of not utilizing ionizing radiation to create medical images, unlike other imaging modalities such as CT and conventional radiography.
Magnetic Field
Some types of medical implants are generally considered contraindications for MRI examinations, while others may be acceptable for patients under high specific MRI conditions. Patients are therefore always asked for complete information about all implants before entering the room for an MRI scan. Several deaths have been reported in patients with pacemakers who have undergone MRI scanning without appropriate precautions. To reduce such risks, implants are increasingly being developed to make them able to be safely scanned, and specialized protocols have been developed to permit the safe scanning of selected implants and pacing devices. Cardiovascular stents are considered safe, however.
Ferromagnetic foreign bodies such as shell fragments, or metallic implants such as surgical prostheses and ferromagnetic aneurysm clips are also potential risks. Interaction of the magnetic and radio frequency fields with such objects can lead to trauma due to movement of the object in the magnetic field or thermal injury from radio-frequency induction heating of the object.
Titanium and its alloys are safe from attraction and torque forces produced by the magnetic field, though there may be some risks associated with Lenz effect forces acting on titanium implants in sensitive areas within the subject, such as stapes implants in the inner ear.
In the United States a classification system for implants and ancillary clinical devices has been developed by ASTM International and is now the standard supported by the US Food and Drug Administration:
- MR-Safe — The device or implant is completely non-magnetic, non-electrically conductive, and non-RF reactive, eliminating all of the primary potential threats during an MRI procedure.
- MR-Conditional — A device or implant that may contain magnetic, electrically conductive or RF-reactive components that is safe for operations in proximity to the MRI, provided the conditions for safe operation are defined and observed (such as 'tested safe to 1.5 teslas' or 'safe in magnetic fields below 500 gauss in strength').
- MR-Unsafe — Nearly self-explanatory, this category is reserved for objects that are significantly ferromagnetic and pose a clear and direct threat to persons and equipment within the magnet room.
There is no evidence for biological harm from even very powerful static magnetic fields.
Peripheral Nerve Stimulation (PNS)
The rapid switching on and off of the magnetic field gradients is capable of causing nerve stimulation. Volunteers report a twitching sensation when exposed to rapidly switched fields, particularly in their extremities. The reason the peripheral nerves are stimulated is that the changing field increases with distance from the center of the gradient coils (which more or less coincides with the center of the magnet). Although PNS was not a problem for the slow, weak gradients used in the early days of MRI, the strong, rapidly switched gradients used in techniques such as EPI, fMRI, diffusion MRI, etc. are indeed capable of inducing PNS. American and European regulatory agencies insist that manufacturers stay below specified dB/dt limits (dB/dt is the change in field per unit time) or else prove that no PNS is induced for any imaging sequence. As a result of dB/dt limitation, commercial MRI systems cannot use the full rated power of their gradient amplifiers.
Heating Caused by Absorption of Radio Waves
Every MRI scanner has a powerful radio transmitter to generate the electromagnetic field which excites the spins. If the body absorbs the energy, heating occurs. For this reason, the transmitter rate at which energy is absorbed by the body has to be limited (see Specific absorption rate).
Acoustic Noise
Switching of field gradients causes a change in the Lorentz force experienced by the gradient coils, producing minute expansions and contractions of the coil itself. As the switching is typically in the audible frequency range, the resulting vibration produces loud noises (clicking or beeping). This is most marked with high-field machines and rapid-imaging techniques in which sound pressure levels can reach 120 dB(A) (equivalent to a jet engine at take-off), and therefore appropriate ear protection is essential for anyone inside the MRI scanner room during the examination.
Cryogens
As described in Physics of Magnetic Resonance Imaging, many MRI scanners rely on cryogenic liquids to enable the superconducting capabilities of the electromagnetic coils within. Though the cryogenic liquids used are non-toxic, their physical properties present specific hazards.
An unintentional shut-down of a superconducting electromagnet, an event known as "quench", involves the rapid boiling of liquid helium from the device. If the rapidly expanding helium cannot be dissipated through an external vent, sometimes referred to as a 'quench pipe', it may be released into the scanner room where it may cause displacement of the oxygen and present a risk of asphyxiation.
Oxygen deficiency monitors are usually used as a safety precaution. Liquid helium, the most commonly used cryogen in MRI, undergoes near explosive expansion as it changes from a liquid to gaseous state. The use of an oxygen monitor is important to ensure that oxygen levels are safe for patient/physicians. Rooms built for superconducting MRI equipment should be equipped with pressure relief mechanisms and an exhaust fan, in addition to the required quench pipe.
Because a quench results in rapid loss of cryogens from the magnet, recommissioning the magnet is expensive and time-consuming. Spontaneous quenches are uncommon, but a quench may also be triggered by an equipment malfunction, an improper cryogen fill technique, contaminants inside the cryostat, or extreme magnetic or vibrational disturbances.
Contrast Agents
The most commonly used intravenous contrast agents are based on chelates of gadolinium. In general, these agents have proved safer than the iodinated contrast agents used in X-ray radiography or CT. Anaphylactoid reactions are rare, occurring in approx. 0.03–0.1%. Of particular interest is the lower incidence of nephrotoxicity, compared with iodinated agents, when given at usual doses—this has made contrast-enhanced MRI scanning an option for patients with renal impairment, who would otherwise not be able to undergo contrast-enhanced CT.
Although gadolinium agents have proved useful for patients with renal impairment, in patients with severe renal failure requiring dialysis there is a risk of a rare but serious illness, nephrogenic systemic fibrosis, which may be linked to the use of certain gadolinium-containing agents. The most frequently linked is gadodiamide, but other agents have been linked too. Although a causal link has not been definitively established, current guidelines in the United States are that dialysis patients should only receive gadolinium agents where essential, and that dialysis should be performed as soon as possible after the scan to remove the agent from the body promptly. In Europe, where more gadolinium-containing agents are available, a classification of agents according to potential risks has been released. Recently, a new contrast agent named gadoxetate, brand name Eovist (US) or Primovist (EU), was approved for diagnostic use: this has the theoretical benefit of a dual excretion path.
Pregnancy
No effects of MRI on the fetus have been demonstrated. In particular, MRI avoids the use of ionizing radiation, to which the fetus is particularly sensitive. However, as a precaution, current guidelines recommend that pregnant women undergo MRI only when essential. This is particularly the case during the first trimester of pregnancy, as organogenesis takes place during this period. The concerns in pregnancy are the same as for MRI in general, but the fetus may be more sensitive to the effects—particularly to heating and to noise. However, one additional concern is the use of contrast agents; gadolinium compounds are known to cross the placenta and enter the fetal bloodstream, and it is recommended that their use be avoided.
Despite these concerns, MRI is rapidly growing in importance as a way of diagnosing and monitoring congenital defects of the fetus because it can provide more diagnostic information than ultrasound and it lacks the ionizing radiation of CT. MRI without contrast agents is the imaging mode of choice for pre-surgical, in-utero diagnosis and evaluation of fetal tumors, primarily teratomas, facilitating open fetal surgery, other fetal interventions, and planning for procedures (such as the EXIT procedure) to safely deliver and treat babies whose defects would otherwise be fatal.
Claustrophobia and Discomfort
Despite being painless, MRI scans can be unpleasant for those who are claustrophobic or otherwise uncomfortable with the imaging device surrounding them. Older closed bore MRI systems have a fairly long tube or tunnel. The part of the body being imaged must lie at the center of the magnet, which is at the absolute center of the tunnel. Because scan times on these older scanners may be long (occasionally up to 40 minutes for the entire procedure), people with even mild claustrophobia are sometimes unable to tolerate an MRI scan without management. Some modern scanners have larger bores (up to 70 cm) and scan times are shorter. This means that claustrophobia could be less of an issue, and more patients may now find MRI to be a tolerable procedure. Nervous patients may find the following strategies helpful:
- Advance preparation
- visiting the scanner to see the room and to practice lying on the table
- visualization techniques
- chemical sedation
- general anesthesia
- Coping while inside the scanner
- having a loved one in the room to hold hands, give reassurance, etc.
- holding a "panic button"
- closing the eyes as well as covering them (e.g., with a washcloth or eye mask)
Alternative scanner designs, such as open or upright systems, can also be helpful where these are available. Though open scanners have increased in popularity, they produce inferior scan quality because they operate at lower magnetic fields than closed scanners. However, commercial 1.5 tesla open systems have recently become available, providing much better image quality than previous lower field strength open models.
For babies and other young children, chemical sedation or general anesthesia are the norm, as these subjects cannot be expected or instructed to hold still during the scanning session. Obese patients and pregnant women may find the MRI machine to be a tight fit. Pregnant women may also have difficulty lying on their backs for an hour or more without moving.
Guidance
Safety issues, including the potential for biostimulation device interference, movement of ferromagnetic bodies, and incidental localized heating, have been addressed in the American College of Radiology's White Paper on MR Safety, which was originally published in 2002 and expanded in 2004. The ACR White Paper on MR Safety has been rewritten and was released early in 2007 under the new title ACR Guidance Document for Safe MR Practices.
In December 2007, the Medicines and Healthcare Products Regulatory Agency (MHRA), a UK healthcare regulatory body, issued their Safety Guidelines for Magnetic Resonance Imaging Equipment in Clinical Use.
In February 2008, the Joint Commission, a US healthcare accrediting organization, issued a Sentinel Event Alert #38, their highest patient safety advisory, on MRI safety issues.
In July 2008, the United States Veterans Administration, a federal governmental agency serving the healthcare needs of former military personnel, issued a substantial revision to their MRI Design Guide, which includes physical and facility safety considerations.
The European Physical Agents Directive
The European Physical Agents (Electromagnetic Fields) Directive is legislation adopted by the European Parliament and Council on 29 April 2004. Originally scheduled to be required by the end of 2008, each individual state within the European Union must include this directive in its own law by the end of 2012. Some member nations passed complying legislation and are now attempting to repeal their state laws in expectation that the final version of the EU Physical Agents Directive will be substantially revised prior to the revised adoption date.
The directive applies to occupational exposure to electromagnetic fields (not medical exposure) and was intended to limit workers’ acute exposure to strong electromagnetic fields, as may be found near electricity substations, radio or television transmitters or industrial equipment. However, the regulations impact significantly on MRI, with separate sections of the regulations limiting exposure to static magnetic fields, changing magnetic fields and radio frequency energy. Field strength limits are given, which may not be exceeded. An employer may commit a criminal offense by allowing a worker to exceed an exposure limit, if that is how the directive is implemented in a particular member state.
The directive is based on the international consensus of established effects of exposure to electromagnetic fields, and in particular the advice of the European Commissions's advisor, the International Commission on Non-Ionizing Radiation Protection (ICNIRP). The aims of the Directive, and the ICNIRP guidelines it is based on, are to prevent exposure to potentially harmful fields. The actual limits in the directive are very similar to the limits advised by the Institute of Electrical and Electronics Engineers, with the exception of the frequencies produced by the gradient coils, where the IEEE limits are significantly higher.
Many Member States of the EU already have either specific EMF regulations or (as in the UK) a general requirement under workplace health and safety legislation to protect workers against electromagnetic fields. In almost all cases the existing regulations are aligned with the ICNIRP limits so that the directive should, in theory, have little impact on any employer already fulfilling the legal responsibilities.
The introduction of the directive has brought to light an existing potential issue with occupational exposures to MRI fields. There are at present very few data on the number or types of MRI practice that might lead to exposures in excess of the levels of the directive. There is a justifiable concern amongst MRI practitioners that if the directive were to be enforced more vigorously than existing legislation, the use of MRI might be restricted, or the working practices of MRI personnel might have to change.
In the initial draft a static field strength limit of 2 T was specified. This has since been removed from the regulations, and whilst it is unlikely to be restored without a strong justification, some restriction on static fields may be reintroduced after the matter has been considered more fully by ICNIRP. The effect of such a limit might be to restrict the installation, operation and maintenance of MRI scanners with magnets of 2 T and stronger. As the increase in field strength has been instrumental in developing higher resolution and higher performance scanners, this would be a significant step back.
Individual government agencies and the European Commission have now formed a working group to examine the implications of the directive on MRI and to try to address the issue of occupational exposures to electromagnetic fields from MRI.
See the original article:
Magnetic Resonance Imaging From Wikipedia, the free encyclopedia.


No comments:
Post a Comment